Article Text
Abstract
Background Probiotics have been shown to be immunomodulatory and can affect antibody responses following vaccination. Several immunisations are associated with suboptimal seroconversion rates leaving a substantial part of the population exposed to infection.
Objectives To evaluate the influence of probiotic supplementation on the immune response of infants following mumps, measles, rubella and varicella vaccination.
Methods A randomised, placebo-controlled, double blinded prospective trial was performed in a cohort of healthy infants. Study subjects were randomly assigned to receive probiotics or placebo for a total of 5 months, starting 2 months prior to vaccination. Antibody levels against vaccine components were measured 3 months after immunisation. Treatment-related and vaccine-related adverse events were recorded.
Findings 47 infants completed the study, 25 in the probiotic group and 22 in the placebo group. There was no statistically significant difference in the number of infants failing to reach protective antibody titres against the different vaccine components (three infants in the placebo group against one in the treatment group for rubella, two each for mumps, four children vs two for measles). When combining all results in both groups, a larger percentage of failures to seroconvert occurred in the placebo group (17% vs 8%, p=0.052), a result of borderline significance. The number of infants needed to treat in order to prevent one failed vaccine component was 12. There was no difference in the rate of treatment related adverse effects between the two groups. There was a significant trend toward fewer vaccine related adverse effects in the treatment group.
Conclusions Oral probiotics given to infants during the period of immunisation do not interfere with the immune response to mumps, measles, rubella and varicella vaccine, and may improve seroconversion rates.
Statistics from Altmetric.com
Introduction
Immunisation is one of the most beneficial and cost-effective disease prevention measures. However, even in countries with maximal childhood immunisation coverage the protective effect is not optimal. For example, the efficacy of a single dose of varicella vaccine has been estimated in different studies to be between 70–85% against infection and 95% against severe disease, leaving a substantial proportion of children susceptible to infection.1 2
Several attempts have been made in the medical literature over the years to define the term ‘probiotics’, but the most complete definition was proposed in 2001 by Schrezenmeir and de Vrese: ‘A preparation of or a product containing viable, defined microorganisms in sufficient numbers, which alter the microflora (by implantation or colonisation) in a compartment of the host and by that exert beneficial health effects in this host’.3 The precise mechanism of action of probiotics is not fully understood, but several animal and human studies have proven immunomodulatory effects involving both the humoral and the cellular components of the host's immune system.4,–,13 Klein et al14 demonstrated a significantly elevated percentage of granulocytes and monocytes showing phagocytic activity in a group of young adults treated with daily probiotics compared to placebo. Gill et al6 showed significantly enhanced serum antibody responses to orally and systemically administered antigens in a group of mice treated with daily probiotics. Several studies have shown an immune-enhancing effect following influenza vaccine in older people treated with probiotics15,–,18 and lately some authors have shown an enhanced antibody response in infants treated with probiotics following Haemophilus influenza b (Hib) and hepatitis B vaccinations.19,–,21
What is already known on this topic
▶ Several immunisations are associated with suboptimal seroconversion rates, leaving part of the population exposed to infection.
▶ Probiotics have immunomodulatory properties influencing both the humoral and the cellular components of the immune system.
What this study adds
▶ Oral probiotics given to infants during the period of immunisation do not interfere with the immune response, and may improve seroconversion rates.
▶ Probiotics may potentially reduce vaccine-related adverse effects in infants.
The main objective of the study was to evaluate whether supplementing the diet with oral probiotics enhances the immune response of infants following routine vaccination against mumps, measles, rubella and varicella (MMRV). Secondary outcomes included vaccine-related and treatment-related adverse effects.
Patients and methods
Study design
The study was a prospective, randomised, parallel, double blind, controlled trial.
Setting
Study participants were recruited at the department of paediatrics, Assaf Harofeh Medical Center in Israel during January 2008 to August 2009. The study protocol was approved by the Institutional Review Board as well as by the National Ethics Committee in Paediatric Trials of the Israeli Ministry of Health (registered as ClinicalTrials.gov number, NCT 00645996).
Study population
All infants aged 8–10 months admitted to the paediatric ward with acute illness, whose guardians intended to follow the recommended immunisation schedule in Israel, were eligible for the study. Exclusion criteria were infants suffering from any chronic conditions resulting in immune depression, infants taking medications affecting the immune system, infants with permanent invasive catheters and those born prematurely (prior to gestational week 35).
Supplementation
The treatment group received a commercial formulation (Altman Probiotic Kid Powder; Altman, Maabarot, Israel) containing a combination of four different microorganisms: Lactobacillus acidophilus, strain ATCC4356, Bifidobacterium bifidum strain DSMZ20082, Bifidobacterium longum ATCC157078 and Bifidobacterium Infantis ATCC15697, with a concentration of 3×109 colony-forming units each. The control group received cornflour, matched in appearance and texture.
Study protocol
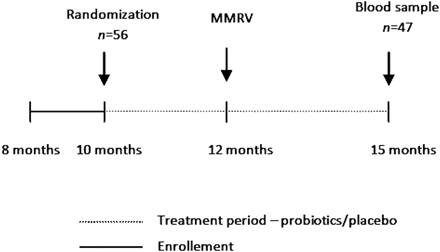
After obtaining written consent, the parents received a box containing 30 sachets of a daily dose of probiotics or placebo (figure 1). Participants were allocated to each group according to a computer generated randomisation list (six block randomisation). Investigators and the parents were blinded to the true content of the sachets. The guardian was asked to supplement the infant's daily intake with the content of a single sachet every day starting at age 10 months. At 12 months of age, children in Israel receive their first dose of live attenuated vaccine against MMRV (Priorix Tetra; GlaxoSmithKline, Auckland, New Zealand). In addition, boosters are given for polio (inactivated polio vaccine), Hib and diphtheria-tetanus-pertussis (DTP). The infants all received their vaccinations, as is customary, at the maternal and child welfare centres of the Israeli ministry of health. Three months after the vaccination a blood sample was collected from the infant and IgG levels against MMRV were measured. During this 5-month period the parents continued supplementation with probiotics/placebo. A research coordinator contacted the parents biweekly to ensure continuing compliance and to address any questions or concerns. During these calls information regarding untoward effects of the treatment was collected. Once monthly the supply of probiotics/placebo was replenished, and the empty sachets were collected, as another method of assessing compliance. Ten days following the immunisation a telephone interview was conducted and information was obtained regarding any untoward vaccine related adverse effects.
Study flow chart. MMRV, mumps, measles, rubella and varicella.
In Israel it is recommended by the Ministry of Health to screen all infants for anaemia around the age of 12 months by performing a complete blood count. We asked the parents to postpone the blood count for 3 months, and tested for vaccine responses at the same time, thereby avoiding exposing the infants to unnecessary painful procedures. The primary outcome of our study was the percentage of infants achieving protective antibody levels against vaccine components in the study group compared to the placebo group. Secondary outcomes included rates of vaccine-related and treatment-related adverse events in the two groups.
Laboratory analysis
The presence of protective levels of IgG antibodies against vaccine components was determined by automated semiquantitative enzyme linked fluorescent assay according to the manufacturer's instructions. Vitek immunodiagnostic assay system (VIDAS) MSG, MPG, VZG and RBG were all analysed on a VIDAS automated immunoanalyser using a two-step enzyme immunoassay sandwich method (bioMerieux SA, Lyon, France). This system is more sensitive than conventional haemagglutination inhibition and indirect fluorescent antibody assays, and has high intra-assay reproducibility. Protective titres were determined by previous reports in the literature,1 2 22 and by the manufacturer's instructions. Optical density values were indexed according to the kit instructions corresponding to the following values: rubella >40 IU/ml, varicella >150 mIU/ml and measles >200 mIU/ml. For mumps, as there is no known protective level of neutralising antibodies, a commonly used cut-off of 40 mIU/ml was used.
Statistics
Assuming a mean difference in the rate of seroconversion of 15% between the treatment and control groups, we estimated a required sample size of 63 participants in each group in order to achieve the required power (85%, type 1 error = 0.05, two tailed test).
Numerical data are expressed using means±SDs and their medians and range. Categorical data are expressed using percentages. In order to compare between the two subgroups we used the t test and the non-parametric Mann–Whitney test for two independent groups for the numerical data, and χ2 test and Fisher's exact test for the categorical data. The p values for the comparison between the two groups are reported. All analyses were performed using SPSS V.14.0.1.
Results
During the trial period we approached 250 eligible participants, but succeeded in recruiting only 56 infants, of whom nine dropped out due to poor compliance (two subjects), refusal to immunise the child (two subjects), treatment-related adverse effects (two subjects with fussiness and abdominal pain, one in each group) and emigrating to another country (one subject). Two other participants gave no reason for the refusal to continue the study (figure 2). Baseline features of study participants were similar in both groups (table 1) and overall protective rates following vaccination were comparable with data from larger studies.
Revised CONSORT flow diagram.
Comparison between study group variables
There was no significant difference between the two groups in the number of infants achieving protective titres of antibodies against each of the four vaccine components. (table 2). When we combined all the different antibody titres from each arm into a single group there was an advantage of borderline statistically significance to the treatment group, in achieving protective titres (p=0.052).
Number of study participants failing to reach protective IgG antibody titres 3 months after vaccination for mumps, measles, rubella and varicella
Comparing the vaccine-related adverse events, as reported by the parents, there was a trend to fewer febrile episodes and fewer cases of diarrhoea in the treatment group (table 3), but the numbers were too small to reach statistical significance. When looking at the overall rate of adverse events there were fewer events in the treatment group (p=0.051). Importantly, there was no difference in the rate of untoward side effects from treatment between the two groups.
Number of study participants reporting vaccine related adverse events up to 10 days following vaccination for mumps, measles, rubella and varicella as reported by the parents during phone interview
Discussion
To the best of our knowledge, this is the first study to evaluate the effect of probiotic supplementation on the antibody titres of infants following MMRV vaccination. Our study shows that probiotic supplementation does not interfere with the immune response of healthy infants to live vaccines. Moreover, we have shown a trend towards a better antibody responses in the probiotic treatment group, with more infants reaching protective titres 3 months after immunisation. This effect is modest, but can have an important impact especially when considering vaccines with suboptimal protective effects, such as varicella or flu. Interestingly, we found no difference in mean antibody concentration as determined by the semiquantitative fluorescence reading. This may suggest that when there is an adequate immune response, the probiotics have no additive effect on the quantity of antibodies produced, but that their value lies in a boosting effect only for infants with a weak immunological response to vaccination. Prior studies have shown that infants in developing countries usually mount higher antibody titres following vaccination, than do infants from developed countries.23 This has been explained by the ‘hygiene hypothesis’ stating that early repeated antigenic stimulation by viral and bacterial infections stimulates a Th1 profile of the immune response, favouring IgG2 production. Improvements in public health and hygiene, and the steady decline in family size in the developed world can thus potentially favour a Th2 response, explaining the reduced ability to mount adequate antibody responses following exposure to infections or vaccination.9 14 24 Accordingly, Pérez et al23 showed recently that probiotic supplementation has no effect on antibody responses following DTP-Hib and 23-valent pneumococcal vaccine in children of low socioeconomic status in Argentina. It is possible that probiotic supplementation is a safe way of ‘closing the gap’ between infants in developed countries and those from developing parts of the world, regarding early microbial colonisation and antigenic stimulation.
Another finding in our current study was the trend to a reduced incidence of vaccine-related adverse events, mainly febrile episodes. This finding is especially important in light of the recent study by Prymula et al25 showing impaired antibody responses in infants who were treated with paracetamol following immunisation. If indeed our results are consistently reproduced in future studies, probiotic supplementation will have the added effect of potentially reducing the need for antipyretic administration after vaccination.
Consistent with the available literature, in our study there was no difference in the rate of reported adverse effects between the treatment groups among infants who completed the study.4 26 Only two subjects dropped out prematurely due to gastrointestinal adverse effects—one infant in each group. It appears that probiotic supplementation is a safe option for healthy infants.
There are several limitations to our study; first, the number of infants recruited was lower than expected. Our primary goal was to reach a cohort of 63 subjects in each group. After 2 years of recruitment we had to terminate the study without reaching our goal, and so even though there are several clear trends, most of our results are not statistically significant. In retrospect, statistical analysis of our results shows that if these trends persist, we would need 65 participants in each group, almost precisely matching our primary assumption. Nevertheless, we still feel our results are valuable, in that despite the sample size they show positive trends. This project is a pilot study and further investigations are needed.
One of the reasons we had difficulty recruiting children was that we enrolled healthy children, whereas most other studies published on the subject have enrolled children suffering from acute (diarrhoea or other infections) or chronic illnesses (prematurity, allergy), thus providing the parents with an incentive to participate.20 21 27,–,29 In most of the clinical studies published on the influence of probiotics on immunisations, the children were part of a larger study examining the influence of probiotics on various conditions—mainly atopy. We feel that this is actually an important advantage of our study, as almost no other authors have shown immunomodulatory effects in healthy children.
Another common possible confounder is the question of compliance. In the current study we took measures to ensure compliance, and as our investigation was blinded and placebo controlled, even if there was some degree of non-compliance it would not have influenced the results.
Finally, previous studies have shown that not all probiotic preparations have the same beneficial effects.30 In our study we chose a common commercial preparation containing four different strains that have all been examined in previously published investigations, although not when given as a combination.
In conclusion, much has been written regarding the immunomodulatory effects of probiotic supplementations. Our current pilot study shows a trend towards higher seroconversion rates following vaccination with MMRV when infants are given daily probiotic supplementation. This effect is modest, and further studies are needed to elucidate the beneficial effects of probiotic preparations on the immune system of infants.
Acknowledgments
This study was supported by a grant from the Materna infant nutrition research institute 2007.
References
Footnotes
-
Competing interests None.
-
Patient consent Obtained.
-
Ethics approval This study was conducted with the approval of the Institutional Review Board, Assaf Harofeh Medical Center, Israel and Ministry of Health Review Board, Israel.
-
Provenance and peer review Not commissioned; externally peer reviewed.